PARTICIPATION OF ADENOSINE SYSTEM IN THE KETAMINE-INDUCED MOTOR ACTIVITY IN MICE
PARTICIPATION OF ADENOSINE SYSTEM IN THE KETAMINE-INDUCED MOTOR ACTIVITY IN MICE, Pol. J. Pharmacol., 2004, 56, 000-000.
Marcin Mandryk, Sylwia Fidecka, Ewa Poleszak, Danuta Malec
Department of Pharmacodynamics, Medical University, Staszica 4, PL 20-081 Lublin, Poland
Running head: Ketamine-induced motor activity and adenosine receptor ligands
The influence of adenosine receptor ligands on ketamine-induced locomotor activity was studied in mice. Ketamine-induced hyperactivity (10 mg/kg) was significantly and dose-dependently attenuated by CGS 21680 (selective A2A receptor agonist), and NECA (A1/A2 adenosine receptor agonist), but not by CPA (selective A1 adenosine receptor agonist). Motor activity produced by subthreshold dose (2.5 mg/kg) of ketamine was significantly increased by DMPX (selective A2A receptor antagonist) and caffeine (A1/A2 adenosine receptor antagonist), but not by DPCPX (selective A1 adenosine receptor antagonist).
These results suggest that adenosinergic system is involved in ketamine-induced motor activity and seem to indicate a predominant role of A2A adenosine receptor in this effect.
Key words: ketamine, adenosine, motor activity, mice
INTRODUCTION
Adenosine is known to play a neuromodulatory role in the central nervous system (CNS) acting via four types of receptors: A1, A2A, A2B and A3 [10]. Adenosine A1 receptors are widely distributed in the brain, with the highest densities in the hippocampus, cerebellum and neocortical areas [5]. Functional studies indicate that the striatal adenosine A1 receptors modulate dopamine release [21]. Moreover, D1 and A1 receptors are present on GABAergic terminals of striatal origin in the substantia nigra, and an inhibitory A1/D1 receptor interaction, with respect to GABAergic striatonigral transmission, has been demonstrated [6, 9]. Anatomical studies have shown that the adenosine A2A receptor subtype has a relatively high concentration in the striatum [17, 35], particularly in the striatopallidal GABAergic neurons [32], where these receptors are co-localized with D2 receptors [8].
The data indicate that adenosine plays an opposite role to dopamine in the brain [6, 7]. Adenosine A2A receptor agonists produce behavioral effects similar to those induced by D2 receptor blockers (neuroleptics), and adenosine A2A antagonists can reverse various dopamine-related motor impairments, such as locomotor suppression, catalepsy or muscular rigidity [4, 16, 26, 33]. In the previous paper we observed that selective adenosine A1 receptor agonist had some attenuating influence on the development of amphetamine dependence in the conditioned place preference test in rats [30].
Some experimental data suggest the possibility of interaction between adenosine, dopamine and excitatory amino acid systems. For example, subconvulsant doses of N-methyl-D-aspartate (NMDA) in mice induce an initial motor depression followed by motor activation [12, 13]. The latter effect of NMDA is partly related to its dopamine-releasing action [13, 18, 36]. However, depressant effects of NMDA are suggested to be mediated by adenosinergic mechanisms since they are antagonized by adenosine receptor antagonist, theophylline [12] and stimulation of NMDA receptor has been shown to increase the striatal extracellular levels of adenosine [3, 27]. Moreover, endogenous adenosine plays a crucial role in neurodegeneration, protects against ischemic or excitotoxic neuronal damage [38].
Ketamine is a phencyclidine analogue with non-competitive NMDA receptor antagonist activity. It is used clinically as a “dissociative anesthetic”. However, emergency use of ketamine anaesthesia is often accompanied by restlessness, mood changes, psychomotor agitation and hallucination in humans [37]. In animals, low doses of ketamine induce locomotor stimulation, which is the behavioral parameter classically considered to mimic psychotic symptoms in humans, while its higher doses are associated with ataxia and stereotyped behaviors [25, 39].
Biochemical data have shown that ketamine enhances dopamine and noradrenaline release [34], and inhibits dopamine [22] and norepinephrine [34] uptake in the striatum and cortex, respectively. It has been suggested that ketamine may have an indirect dopamine agonist activity, and ketamine-induced behavioral stimulation may be connected with the dopamine system [19]. Moreover, the motor activating effects of phencyclidine, another non-competitive NMDA receptor antagonist, were dose-dependently inhibited by CGS 21680, selective A2A adenosine receptor agonist [31].
The abovementioned dopamine-adenosine interactions seem to indicate that the ketamine-induced motor activity may be modulated by the ligands of adenosine receptors. For that reason, the effects of adenosine receptor agonists and antagonists on ketamine-induced motor activity were estimated in the present studies.
MATERIALS and METHODS
Animals
The experiments were carried out on male albino Swiss mice (18-28 g). The animals were kept 8-10 to a cage at room temperature of 20 ± 1ºC and under a 12 h light/dark cycle. Standard food (Murigran pellets, Bacutil, Motycz, Poland) and tap water were available ad libitum. All experiments were performed between 9 a.m. and 3 p.m.
The experiments were performed in accordance with the opinion of Local Ethics Committee.
Drugs
The following drugs were used: adenosine receptors agonists: N6- cyclopentyladenosine (CPA) – A1 receptor agonist, 2-p-(carboxyethyl) phenethyl amino-5’-N-ethylcarboxamidoadenosine (CGS 21680) – A2A receptor agonist, 5’-N-ethylcarboxamidoadenosine (NECA) – A2/A1 adenosine receptor agonist (all from RBI, USA); a adenosine receptors antagonists: 8-cyclopentyl-1, 3-dimethylxanthine (DPCPX) – A1 receptor antagonist and 3, 7-dimethyl-1-propargylxanthine (DMPX) – A2 receptor antagonist (both RBI, USA), caffeine – a nonselective adenosine receptor antagonist (Polfa, Poland), and NMDA receptor antagonists: ketamine (Ketanest, Parke-Davis, Germany).
All drugs were dissolved in saline. Adenosine receptor ligands and ketamine were administered intraperitoneally (ip). Control animals received the same volumes of saline.
Procedure
Locomotor activity was measured in mice always 10 min after the injection of adenosine ligands and immediately after the ketamine administration. The animals were placed singly in round actometer cages (32 cm in diameter, two light beams) for 30 min and motor activity was recorded without any acclimatization period.
Ketamine was used at the following doses: 2.5 mg/kg – the subthreshold dose, and 10 mg/kg – the dose increasing significantly locomotor activity in comparison with the control (saline) group.
The results, presented in figures, include total activity count from 30 min period.
Statistics
Results are shown as means ± SEM. Statistical significance of differences between the groups was determined by Student’s t-test; p values <0.05 were considered as statistically significant.
.
RESULTS
Effect of adenosine receptor agonists on ketamine-induced hypermotility in mice
Ketamine, given alone at the dose of 10 mg/kg, significantly increased the locomotor activity of mice (Fig. 1A, B and C).
Locomotor activity in mice was significantly decreased by all adenosine receptor agonists. CPA, given alone, reduced motor activity dose-dependently, and statistically significant effect was observed at the dose of 0.05 mg/kg (Fig. 1A). CGS 21680 significantly decreased the motor activity only at the higher dose of 0.1 mg/kg (Fig. 1B). NECA, given at the dose of 0.005 and 0.01 mg/kg, significantly but not dose-dependently diminished motility of mice (Fig. 1C).
Hypermotility induced by ketamine (10 mg/kg) was significantly decreased by the higher dose (0.1 mg/kg) of CGS 21680 (Fig. 1B) but not by CPA (Fig 1A). NECA (adenosine A2/A1 agonist) significantly and dose-dependently reduced ketamine hyperactivity (Fig. 1C).
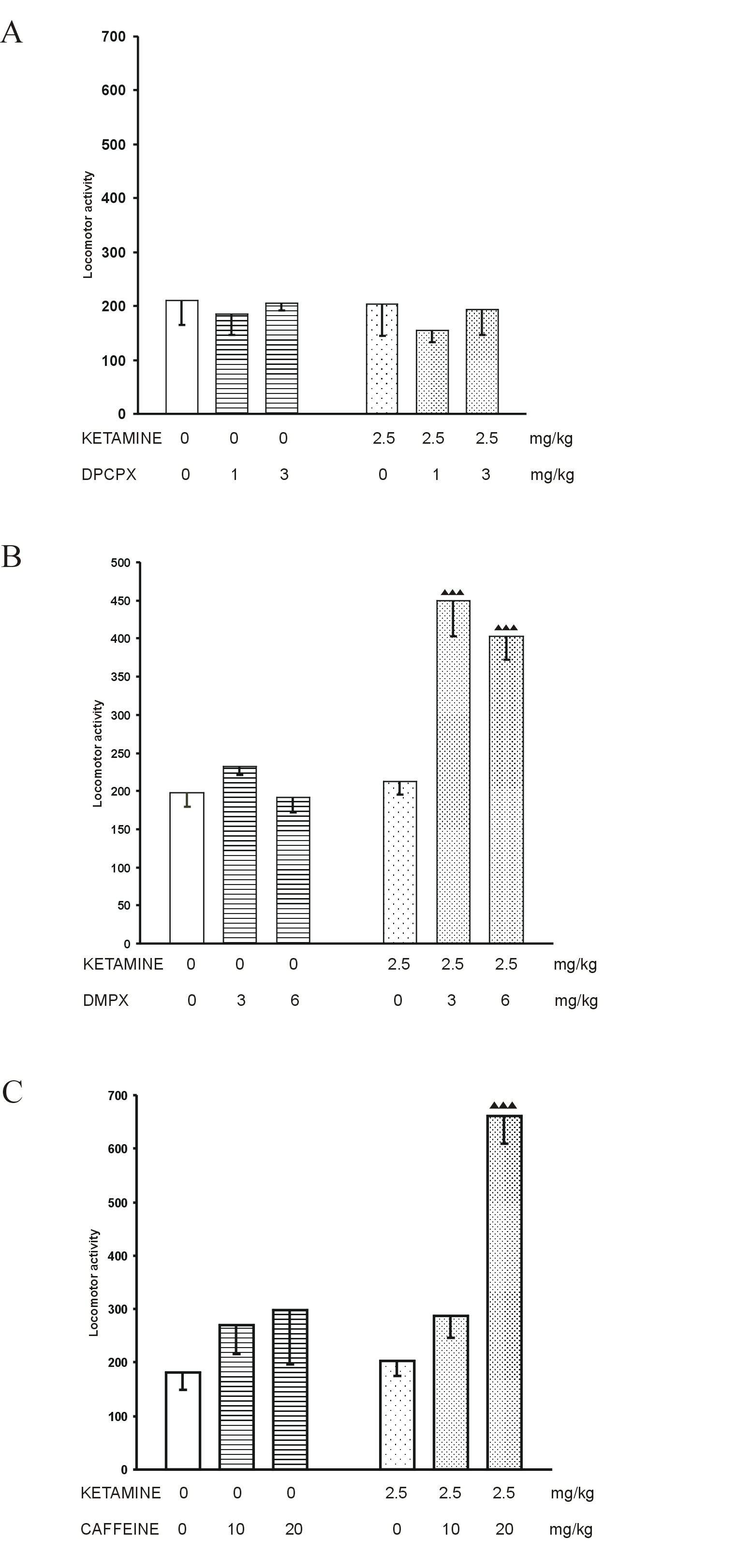
Effect of adenosine receptor antagonists on motility produced by the subthreshold dose of ketamine
Locomotor activity in mice was not changed by ketamine, given at the subthreshold dose of 2.5 mg/kg (Fig. 2A, B and C).
Adenosine receptor antagonists DPCPX (1 and 3 mg/kg) and DMPX (3 and 6 mg/kg), given alone, did not change the motility of control mice (Fig.2A and B, respectively). Caffeine (10 and 20 mg/kg), given alone, slightly but not significantly enhanced the locomotor activity of saline-treated mice (Fig. 2C).
DPCPX (1 and 3 mg/kg) did not influence the locomotor activity in mice evoked by the subthreshold dose of ketamine (Fig. 2A), whereas DMPX (3 and 6 mg/kg) and caffeine (20 mg/kg) significantly increased this ketamine action (Fig. 2B and C).
DISCUSSION
Our present results have shown that ketamine-induced motor activity is influenced by the adenosinergic system. In this paper we confirm that ketamine administered at small, subanesthetic dose of 10 mg/kg induces hyperactivity in mice. It is well known that dopaminergic mechanisms play an important role in mediating the locomotor activity. Ketamine has been shown to exert its action via multiple mechanisms. Apart from being a non-competitive NMDA receptor antagonist, it may influence dopamine transmission and receptors. For example, some reports demonstrate that ketamine inhibits uptake and enhances the release of dopamine [19], and hyperlocomotion induced by this drug is potently antagonized by the low dose of haloperidol, a dopamine receptor antagonist, in mice [20] and rats [39]. Moreover, Kapur and Seeman [23, 24] have found that ketamine shows an affinity for dopamine D2 receptor as strong as for the NMDA receptor.
These results indicate that interactions between dopamine and NMDA receptors are involved in hyperlocomotion produced by ketamine. However, our present experiments with adenosine receptor ligands have shown that the adenosine system is also involved in ketamine-induced hypermotility of mice. Namely, a motor activating effect of ketamine (10 mg/kg) was inhibited by CGS 21680 (A2A receptor agonist) and NECA (A1/A2 receptor agonist) but not by CPA, selective A1 receptor agonist. It means that A2A adenosine receptors play a more important role in ketamine hyperactivity.
Adenosine and related compounds can induce such effects as depression of motor behavior, motor deficiences, anticonvulsant actions and analgesia [2]. Motor depressant effects were observed after administration of both A1 and A2A adenosine receptor agonists, however, A2A receptors seem to be more involved in the modulation of motor behavior [28]. A2A receptors are selectively expressed and co-localized with dopamine D2 receptors in the striatopallidal neurons [8, 32]. It has been shown that adenosine A2A receptors interact directly with dopamine D2 receptors in the striatopallidal neurons, and that stimulation of A2A receptors can decrease the affinity of dopamine for D2 receptors [11]. Our findings confirm that adenosine receptor agonists produce motor depressing effects, and they antagonize ketamine-induced hyperactivity mainly by the stimulation of A2A receptor.
In contrast to a depressant effect of adenosine agonist on motor behavior, the antagonisms of adenosine receptors, especially of sites A2A [14], produce motor stimulant effects, particularly in animals with dopamine hypofunction [15, 28]. In our experiments, motor behavior of mice was not significantly changed by adenosine receptor antagonists, given alone at the used doses. Nevertheless, the DMPX, a selective A2 adenosine receptor antagonist, significantly increased locomotor effects produced by the subthreshold dose (2.5 mg/kg) of ketamine. Intensification of the motor activity was also observed after administration of caffeine at the dose of 20 mg/kg. Similarly to the lack of effects of CPA (A1 receptor agonist) on motor activating effects of ketamine, the blockade of A1 adenosine receptor by DPCPX did not influence the motor effects of the threshold dose of ketamine. It means that A1 adenosine receptors do not play an important role in ketamine-induced motor activity. Our finding that A2A receptor is involved in control of motor activity is in line with the reports of other authors. Nagel et al.[28] have shown that adenosine A2A receptor antagonisms lead to stimulation of behavioral activity, and selective A2A antagonists are able to reverse dopamine -receptor blockade- mediated catalepsy [16, 26] and locomotor suppression [4]. Moreover, blockade of A2A receptor alleviates the impaired locomotion in the dopamine D2 receptor-deficient mice [1], and these results show that adenosine A2A and dopamine D2 receptors have antagonistic and independent activities in controlling neuronal and motor functions. Nash and Brotchie [29] have shown that NMDA-induced increase in striatal cAMP was completely blocked by DMPX. It means that NMDA receptors modulate cAMP levels via adenosine A2A receptors within the striatum.
Summing up, our results have shown that the adenosinergic system is involved in ketamine-induced motor activity and seem to indicate a predominant role of A2A adenosine receptors in this effect: adenosine A2A receptor agonists decrease ketamine-induced hyperactivity, and adenosine A2A receptor antagonists increase the motor effects of subthreshold dose of ketamine. It means that, apart from NMDA and dopamine receptors, also adenosine A2A receptors are involved in ketamine-induced motor activity.
REFERENCES
1. Aoyama S, Kase H, Borrelli E: Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an adenosine A2A receptor antagonist. J Neurosci, 2000, 20, 5848-5852.
2. Barraco RA: Behavioral actions of adenosine and related substances. In: Adenosine and Adenine Nucleotides as Regulators of Cellular Function. Ed. Phillis JW, CRC Press, Boca Raton, FL, 1991, 339-366.
3. Chen Y, Graham DI, Stone T: Release of endogenous adenosine and its metabolites by activation of NMDA receptors in the rat hippocampus in vivo. Br J Pharmacol, 1992, 106, 632-638.
4. Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, Salamone JD: The adenosine A2A antagonist KF17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res, 2004, 148, 47-54.
5. Fastbom J, Pazos A, Palicios JM: The distribution of adenosine A1 receptors and 5’-nucleotidase in the brain of some commonly used experimental animals. Neuroscience, 1987, 22, 813-826.
6. Ferré S: Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology, 1997, 133, 107-120.
7. Ferré S, Fuxe K, von Euler G, Johansson B, Fredholm BB: Adenosine-dopamine interactions in the brain. Neuroscience, 1992, 51, 501-512.
8. Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack A, Adler EM, Reppert SM: Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res, 1992, 14, 186-195.
9. Floran B, Barajas C, Floran L, Erlij D, Aceves J: Adenosine A1 receptors control dopamine D1-dependent [3H] GABA release in slices of substantia nigra pars reticulata and motor behavior in rat. Neuroscience, 2002, 115, 743-751.
10. Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J: International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacol Rev, 2001, 53, 527-552.
11. Fuxe K, Ferre S, Zoli M, Agnati LF: Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Brain Res Rev, 1998, 26, 258-273.
12. Giménez-Llort L, Martínez E, Ferré S: Different effect of dopamine antagonists on spontaneous and NMDA-induced motor activity in mice. Pharmacol Biochem Behav, 1997, 56, 549-553.
13. Giménez-Llort L, Martínez E, Ferré S: Dopamine-independent and adenosine-dependent mechanisms involved in the effects of N-methyl-D-aspartate on motor activity in mice. Eur J Pharmacol, 1995, 275, 171-177.
14. Griebel G, Saffroy-Spittler M, Misslin R, Remmy D, Vogel E, Bourguignon JJ: Comparison of the behavioural effects of an adenosine A1/A2-receptor antagonist, CGS 5943A, and an A1-selective antagonist, DPCPX. Psychopharmacology, 1991, 103, 541-544.
15. Hauber W, Nagel J, Sauer R, Müller CE: Motor effects induced by a blockade of adenosine A2A receptors in the caudate-putamen. Neuroreport, 1998, 9, 1803-1806.
16. Hauber W, Neuscheler P, Nagel J, Müller CE: Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A2a receptors in the caudate-putamen of rats. Eur J Neurosci, 2001, 14, 1287-1293.
17. Hettinger BD, Lee A, Linden J, Rosin DL: Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat stratium. J Comp Neurol, 2001, 431, 331-346.
18. Imperato A, Scrocco MG, Bacchi S, Angelucci L: NMDA receptors and in vivo dopamine release in the nucleus accumbens and caudatus. Eur J Pharmacol, 1990, 187, 555-556.
19. Irifune M, Shimizu T, Nomoto M: Ketamine-induced hyperlocomotion associated with alteration of presynaptic component of dopamine neurons in the nucleus accumbens of mice. Pharmacol Biochem Behav, 1991, 40, 399-407.
20. Irifune M, Shimizu T, Nomoto M, Fukuda T: Involvement of N-methyl-D-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice. Pharmacol Biochem Behav, 1995, 51, 291-296.
21. Jin S, Johansson B, Fredholm BB: Effects of adenosine A1 and A2 receptor stimulation on electrically evoked dopamine and acetylcholine release from rat striatal slices. J Pharmacol Exp Ther, 1993, 267, 801-808.
22. Johnson KM, Snell LD: Effects of phencyclidine (PCP)-like drugs on turning behaviour, 3H-dopamine uptake, and 3H-PCP binding. Pharmacol Biochem Behav, 1985, 22, 731-735.
23. Kapur S, Seeman P: Ketamine has equal affinity for NMDA receptors and the high-affinity state of the dopamine D2 receptor. Biol Psychiatry, 2001, 49, 954-957.
24. Kapur S, Seeman P: NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D2 and serotonin 5-HT2 receptors - implications for models of schizophrenia. Mol Psychiatry, 2002, 7, 837-844.
25. Lapin IP, Rogawski MA: Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice. Behav Brain Res, 1995, 70, 145-151.
26. Malec D: Haloperidol-induced catalepsy is influenced by adenosine receptor antagonists. Pol J Pharmacol, 1997, 49, 323-327.
27. Melani A, Corsi C, Giménez-Llort L, Martínez E, Ögren SO, Pedata F, Ferré S: Effect of N-methyl-D-aspartate on motor activity and in vivo adenosine striatal outflow in the rat. Eur J Pharmacol, 1999, 385, 15-19.
28. Nagel J, Schladebach H, Koch M, Schwienbacher I, Muller CE, Hauber W: Effects of an adenosine A2A receptor blockade in the nucleus accumbens on locomotion, feeding, and prepulse inhibition in rats. Synapse, 2003, 49, 279-286.
29. Nash JE, Brotchie JM: A Common Signaling Pathway for Striatal NMDA and Adenosine A2a Receptors: Implications for the Treatment of Parkinson's Disease. J Neurosci, 2000, 20, 7782-7789.
30. Poleszak E, Malec D: Effects of adenosine receptor agonists and antagonists in amphetamine-induced conditioned place preference test in rats. Pol J Pharmacol, 2003, 55, 319-26.
31. Rimondini R, Ferre S, Ogren SO, Fuxe K: Adenosine A2A agonists: a potential new type of atypical antipsychotic. Neuropsychopharmacology, 1997, 17, 82-91.
32. Schiffman SN, Jacobs O, Vanderhaeghen JJ: The striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephaline but not by substance P neurons: an in-situ hybridization study. J Neurochem, 1991, 57, 1062-1067.
33. Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, Kuwana Y: Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology, 1999, 147, 90-95.
34. Smith DJ, Azzaro AJ, Zaldivar SB, Palmer S, Lee HS: Properties of the optical isomers and metabolites of ketamine on the high affinity transport and catabolism of monoamines. Neuropharmacology, 1981, 20, 391-396.
35. Svenningsson P, Le Moine C, Fisone G, Fredholm BB: Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol, 1999, 59, 355-396.
36. Svensson L, Zhang J, Johannessen K, Engel JA: Effect of local infusion of glutamate analogues into the nucleus accumbens of rats: an electrochemical and behavioural study. Brain Res, 1994, 643, 155-161.
37. Vargiu L, Stefanini E, Musinu C, Saba G: Possible role of brain serotonin in the central effects of ketamine. Neuropharmacology, 1978, 17, 405-408.
38. Wardas J: Neuroprotective role of adenosine in the CNS. Pol J Pharmacol, 2002, 54, 313-26.
39. Yamamoto M, Mizuki Y, Suetsugi M, Ozawa Y, Ooyama M, Suzuki M: Effects of dopamine antagonists on changes in spontaneous EEG and locomotor activity in ketamine-treated rats. Pharmacol Biochem Behav, 1997, 57, 361-365.
List of figures
Fig. 1. The influence of adenosine receptor agonists: A. CPA, B. CGS 21680 and C. NECA on ketamine-induced hyperactivity in mice. Each bar represents the mean ± SEM for a group of 10 mice. * p<0.05, ** p<0.01, *** p<0.001 vs. saline, p<0.01, p<0.001 vs. ketamine + saline (Student’s t-test)
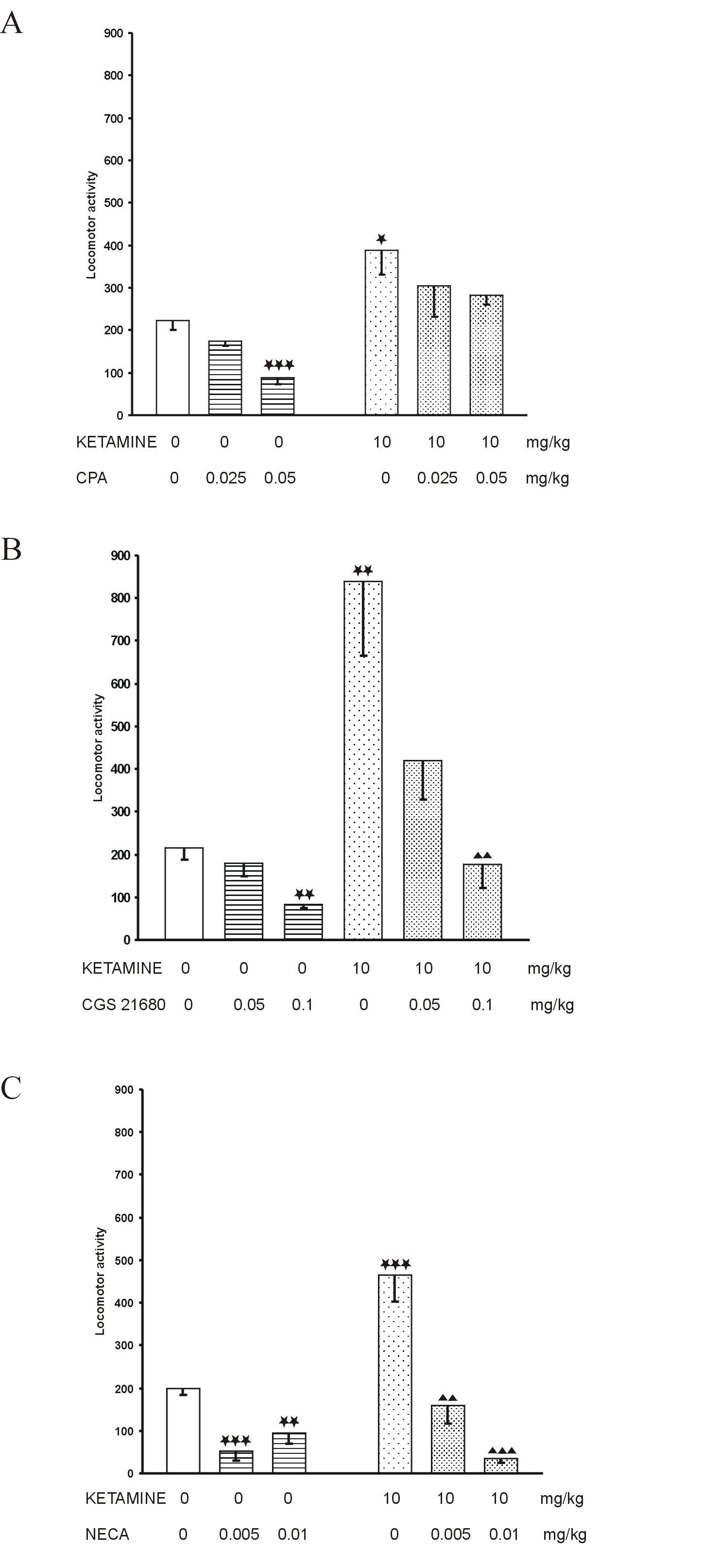
Fig. 2. The influence of adenosine receptor antagonists: A. DPCPX, B. DMPX and C. caffeine on locomotion induced by the threshold dose of ketamine in mice. Each bar represents the mean ± SEM for a group of 10 mice. p<0.001 vs. ketamine + saline (Student’s t-test)